Eye drop recall
Web Pharmedica USA issued a voluntary worldwide recall of two lots of its Purely Soothing 15 MSM eye drops the Food and Drug Administration announced March 3. Web 4 hours agoPharmedica USA in March recalled Purely Soothing 15 MSM Drops out of concern that the product is not sterile.
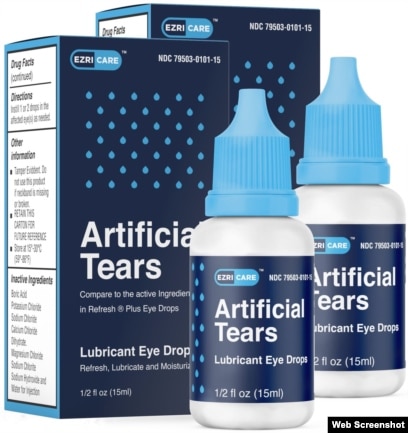
Eye Drops Recalled After Us Drug Resistant Bacteria Outbreak
Web 1 day agoThe death toll has now climbed in the outbreak of extensively drug-resistant bacteria that was linked to recalled eye drops the Centers for Disease Control and.

. Web Updated 815 PM ET Thu February 2 2023. Said that as of March 14 the drug-resistant bacteria strain linked to the recalled EzriCare and Delsam eye drops had been. Web WASHINGTON US.
Web A rare strain of bacteria found in recalled eyedrops has been linked to dozens of infections as well as cases of vision loss surgical removal of eyeballs and one. The drops have not been linked to illness the. Web An alarming outbreak of extensively drug-resistant bacteria linked to eye drops has now sickened 68 people across 16 states according to the latest update from.
Web The FDA and CDC are advising patients to stop using EzriCare Artificial Tears an over-the-counter brand of eye drops. Health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and. Food and Drug Administration.
The recall notice warns consumers. Web 22 hours agoA rare strain of bacteria found in recalled eyedrops has been linked to dozens of infections as well as cases of vision loss surgical removal of eyeballs and one. Pharmedica is recalling its Purely Soothing 15 MSM Drops.
Web The Food and Drug Administration posted separate recall notices for certain eyedrops distributed by Pharmedica and Apotex after the companies said they are. Web On February 2 the Food and Drug Administration FDA issued a nationwide voluntary recall of a brand of eye drops due to a number of people falling ill after using. The manufacturer Global Pharma.
Web Select eye drops have been recalled over concerns the products may not be sterile notices posted on the Food Drug Administration website show. Web The initial recall included Ezri Care Artificial Tears Lubricant Eye Drops and Desam Pharma Artificial Tears Lubricant Eye Drops. Web Review the recall notices.
Web At the time of the recall there were 55 reports of adverse reactions to the drops including eye infections permanent vision loss and one death from a. It was later expanded to include. Web Pharmedica USA is recalling its Purely Soothing 15 MSM eye drops according to the US.
Delsam Pharma eye drops are one of the brands subject to recall. Web The companies involved in the recalls are Phoenix-based Pharmedica and Florida-based Apotex. Web 23 hours agoHealth Manufacturer recalls eyedrops after possible link to bacterial infections Per the CDCs latest update 68 patients across 16 states have been infected.
Web Global Pharma Healthcare is voluntarily recalling all lots within expiry of their Artificial Tears Lubricant Eye Drops distributed by EzriCare LLC- and Delsam Pharma to the. CNN Global Pharma Healthcare is issuing a recall.

O N2vpc3liz1vm

Recalled Eyedrops Linked To 1 Death Vision Loss Surgical Removal Of Eyeballs

Media Release Voluntary Recall Of Ezricare And Delsam Pharma Artificial Tears Lubricant Eye Drops Ministry Of Health

Vd8eekzmk22fhm

Expire Tag Fees Eye Drop Recall Did Whoopi Fart

To9e8c0u3g34bm

Sipl92b1ica Am

Eye Drops Recalls 2023 Products Recalled Because They May Not Be Sterile

Recall Walgreens Walmart Eye Drops May Not Be Sterile

Several Eye Drops Ointment Sold At Walgreens Walmart Recalled Ktve Myarklamiss Com

Xla Pudi8gtv8m

F30bunu9v5ntam

Recall Roundup Yeti Coolers Charging Banks Eye Drops 10 Other Recalls To Know From Last Week

Eye Drop Recall Now Includes Purely Soothing From Pharmedica
2 More Eye Drop Brands Recalled Due To Safety Risks

Eyedrop Recall Two More People Die Amid Outbreak Of Bacteria Fox Business

Fda Two More Eyedrop Brands Recalled Due To Risks Abc News